Who hasn’t heard about the gut-brain axis in recent years? The gut is considered the second brain and the gut microbiota is all over the media. The microbiota, or microbiome, appears to be a key player in the gut-brain axis.1
The term axis applies perfectly in this case since the gut transmits information to the brain and vice versa – this is what is referred to as bidirectional communication.2–4 While this idea is not new and dates back to at least ancient Greece (notably the work of Hippocrates), recent studies illustrate these long-established anatomical observations. In particular, links have been found between psychiatric or neurodegenerative disease and gastrointestinal disease in cohorts of patients. Some studies also suggest that mood, anxiety, or sensitivity to stress may be correlated with the frequency and intensity of abdominal pain or bowel movements.5,6
But what is actually happening on a biological level? It seems easy to grasp that the brain controls intestinal function since it also orchestrates the functioning of all the other organs. From an anatomical point of view, the most obvious communication pathway is the neural one. Indeed, the brain, through its connections with the spinal cord, as well as via the vagus nerve (which connects almost all the internal organs to the central nervous system), is directly connected to the gut. It has been known for a long time that these nerves control the activity of certain muscles, thereby participating in digestive function. This pathway is extremely fast – approximately one second. More recently, research has shown that the neurons that make up these nerves are capable of detecting, transmitting, and interpreting local signals such as the amount of nutrients, as well as the quantity and nature of the microorganisms that compose the microbiota.7,8 On the other hand, it is obvious that the gut and brain can also communicate in a more indirect way through the bloodstream. Any molecule that circulates in the blood will travel through the whole body to the gut and brain. This pathway is slower than the previous one, roughly one minute. Again, it has been scientifically established that the brain can act on the internal organs in the same way as on the muscles by producing hormones, which are released into the blood. Similarly, a signal such as the amount of nutrients ingested during a meal can be transmitted to the brain when the nutrient itself, circulating in the blood, is recognized directly in the brain.
But how can this affect our moods and even our behavior? An enormous number of molecules are found in the blood, some of which come from the gut. These molecules include hormones, of course, but also factors produced by the immune system in contact with our famed microbiota. ut that’s not all: the microbiota itself is capable of producing a variety of molecules, some of which end up in our bloodstream.9 Recent studies even show that our brain has receptors whose specific function is to recognize these microbial molecules.10
So, while we may still be far from understanding how the microbiota can affect our brain in complex and multiple ways, the various communication pathways are now being studied from every angle both in humans and laboratory animal models. One thing is certain: like in all biological systems, communication between the gut and brain is based on a harmonious but fragile balance that, when disrupted, can compromise an individual’s health.
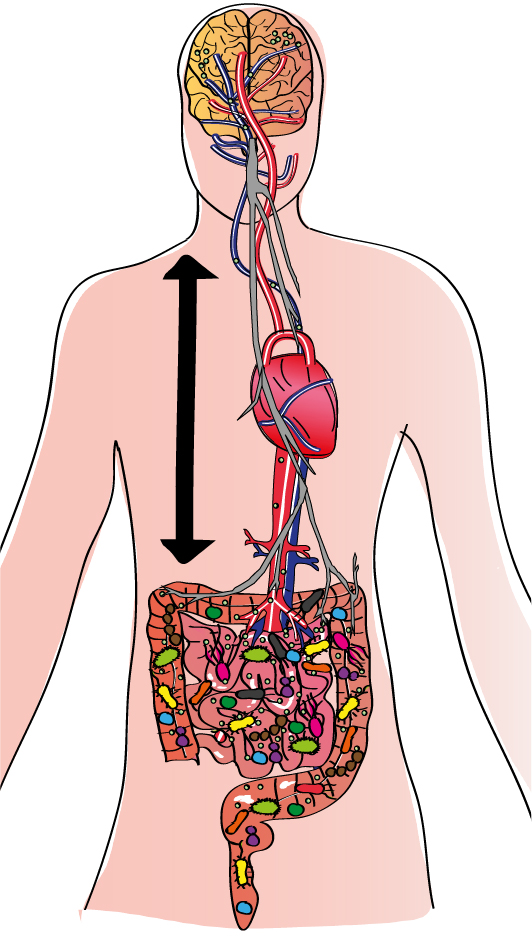
The gut-brain axis
There are multiple pathways, direct and indirect, via which the brain and gut can communicate bidirectionally. The two major pathways, the neural pathway (the vagus nerve, shown in gray) and the bloodstream (the arterial network, in blue, and the venous network, in red) physically link these two organs despite the distance between them. As a result, any signal produced in the gut can potentially be detected by the brain, and vice versa. he molecules released by the microbiota (nutrients or fragments of bacteria, for example, represented by small green circles) can be detected directly, generating various responses. However, this gut-brain communication is much more complex because it involves not only these two organs but all our body’s systems. Consequently, the immune system, which is particularly active in the gut, plays an important intermediary role. Extremely familiar with our microbiota, the immune system is able to detect any changes in it. Immune cells activated by contact with microorganisms can also produce molecules that stimulate the vagus nerve or travel through the bloodstream to the brain. he cells themselves can also follow this route. Furthermore, recent research suggests that our cells can “remember” signals from the microbiota via epigenetic mechanisms, indicating that these signals can have long-term effects.
Bibliography
- Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F. & Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 34, 15490–15496 (2014).
- Grenham, S., Clarke, G., Cryan, J. F. & Dinan, T. G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2, (2011).
- Mayer, E. A. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 12, 453–466 (2011).
- Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
- Folks, D. G. The interface of psychiatry and irritable bowel syndrome. Curr. Psychiatry Rep. 6, 210–215 (2004).
- Buie, T. et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 125 Suppl 1, S1-18 (2010).
- Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
- Bonaz, B. L. & Bernstein, C. N. Brain-Gut Interactions in Inflammatory Bowel Disease. Gastroenterology 144, 36–49 (2013).
- Capuron, L. & Miller, A. H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 130, 226–238 (2011).
- Arentsen, T. et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 22, 257–266 (2017).